3 MicroRNAs and other non-coding RNAs
Table of contents
- 3.1 Thousands of miRNAs have been identified since the first miRNA discovery of lin-4 in 1993
- 3.2 MicroRNA biogenesis involves multiple steps
- 3.3 RNA interference is the central mechanism for gene regulation by miRNAs and small interfering RNA
- 3.4 MicroRNAs have various regulatory roles that are associated with important physiological and pathological processes
- 3.5 Multiple properties of miRNA target recognition may enhance target efficacy in animal
- 3.6 MicroRNA binding also occurs outside of the 3’ UTR
- 3.7 Some of coding and non-coding pairs of cis-NATs potentially may have regulatory interactions with miRNAs
- 3.8 Chromatin associated RNAs are potentially associated with the modification of chromatin structure
- References
Since our main goal is to reveal the miRNA regulation and characteristics, this chapter introduces several different aspects of miRNAs, such as the history of miRNA discovery, miRNA biogenesis, and mechanism of miRNA regulation. In addition to miRNAs, it also describes several other classes of ncRNAs and their potential interactions with miRNAs. Specifically, analysis on such ncRNAs is the main objective of the third sub-goal: miRNA and other ncRNAs.
3.1 Thousands of miRNAs have been identified since the first miRNA discovery of lin-4 in 1993
In 1993, Lee et al. found that lin-4, a gene involved in development timing in C. elegans, produces a small ncRNA instead of a messenger RNA (mRNA) [1]. lin-4 was known to regulate lin-14, but the protein product of lin-4 had been undetected. The result of an alignment analysis indicated that lin-4 has multiple complementary sites on the 3’ UTR of lin-14 [1]. Further experiment revealed that this small ncRNA produced by lin-4 can directly suppress the expression of lin-14 by base-paring on the 3’ UTR. This was the first discovery of this functional ncRNA with about 22 nucleotides that regulates specific protein expression by base-paring on the 3’ UTR of its target mRNA [2]. However, there were no other lin-4-like small ncRNAs identified, and this peculiar regulation of lin-4 was recognized as a rare case [2].
Meanwhile, Fire et al. reported that they observed a gene silencing effect after injecting double-stranded RNAs (dsRNAs) into C. elegans [3] in 1998. They coined the term, RNA interference (RNAi), to describe this gene-silencing mechanism. Soon thereafter, RNAi was found to silence genes at the post-transcriptional level with small RNA molecules with 20-25 nucleotides, called small interfering RNAs (siRNAs) [4,5,6].
Then, nearly seven years after the discovery of lin-4, Reinhart et al. identified a lin-4-like small ncRNA, let-7, in C. elegans in 2000 [7]. They revealed that let-7 is also an ncRNA with about 22 nucleotides that regulates specific protein expression by base-paring on the 3’ UTR. Since let-7 has homologs in various species, this discovery led to identification of many other let-7- and lin-4-like small ncRNAs in other animals, including human and Drosophila [8]. The term microRNA (miRNA) was coined to refer to these small ncRNAs of about 22 nucleotide length [9,10,11]. Moreover, like siRNAs, miRNAs appeared to use the RNAi pathway to regulated genes at the post-transcriptional level [2].
Today, miRNAs are recognized as a very common class of ncRNAs that can regulate protein expression [9,10,11,2]. For instance, miRBase [12,13,14,15], which is the main database for miRNA annotations, contains 15172 entries in 142 species as of release 16, 2010. They are abundant and found mostly in eukaryotes as well as in some viruses. MicroRNAs are known to play many important regulatory roles in eukaryotes [2], whereas some viruses encode viral miRNA genes that potentially regulate fundamental cellular processes both in the viruses and in their host cells [16,17].
3.2 MicroRNA biogenesis involves multiple steps
Although the precise mechanism of miRNA biogenesis is unknown, Figure 3.1 shows the most widely accepted view of the miRNA biogenesis to date. The biogenesis involves multiple processes both in the nucleus and the cytoplasm.
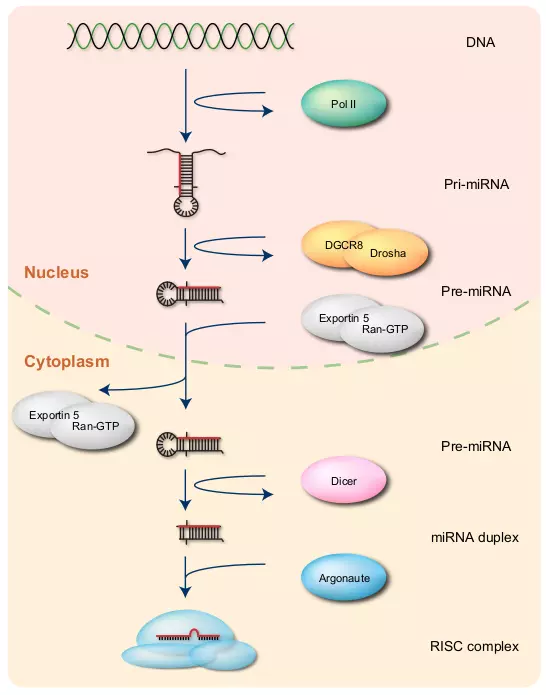
Figure 3.1. MicroRNA biogenesis.
The figure shows the overview of miRNA biogenesis. Pol II transcribes ssRNA from DNA. The ssRNA forms pri-miRNA, that is further processed to a hairpin loop structure called pre-miRNA by DGCR8 and Drosha. Exportin 5 together with Ran-GTP exports pre-miRNA into the cytoplasm. Dicer cleaves pre-miRNA to form miRNA duplex. Only one strand of the miRNA duplex is usually bound to the Argonaute protein and loaded into the RISC complex.
First, RNA polymerase II (Pol II) transcribes a miRNA gene on the chromosome from DNA to single-stranded RNA (ssRNA) with 5’ cap and poly-A tail [18]. This ssRNA called primary miRNA (pri-miRNA) can be several hundreds or thousands nucleotides long, and it may contain one or more hairpin loops [19]. An enzyme called DiGeorge Syndrome Critical Region 8 (DGCR8) recognizes the hairpin loop in pri-miRNA, and a DGCR8 associated enzyme, called Drosha, cleaves the hairpin from the pri-miRNA [20,21,22,23,24]. This cleavage results in a hairpin structure with approximately 60 nucleotides called precursor miRNA (pre-miRNA) [25,20,26,27]. Exportin 5 together with Ran-GTP exports pre-miRNAs from the nucleus to the cytoplasm [28,29].
In the cytoplasm, a Ribonuclease (RNase) III enzyme, called Dicer, cleaves the loop of pre-miRNA and produces a miRNA:miRNA* duplex with approximately 22 nucleotides [20]. Only one strand of this duplex usually becomes a mature miRNA as a guide to the target mRNA, and the other strand, defined as miRNA star-strand or miRNA* [10], is eventually degraded [2]. The mechanism of the strand selection is unclear, but a strand that is less thermodynamically stable at its 5’ end appears to be favored in some cases [30,31]. Argonaute (Ago) proteins are key proteins for miRNA targeting [32]. Ago2, which is one of the Ago clade proteins, is mainly associated with mature miRNAs in mammals [33]. A protein complex called RNA-Induced Silencing Complex (RISC) [34] that incorporates Ago2, uses the mature miRNA as a guide to bind and then catalyze specific target mRNAs [33].
Moreover, there are several known alternative pathways for miRNA biogenesis. For instance, some intronic miRNAs bypass Drosha processing by directly forming a pre-miRNA-like hairpin structure. These intronic miRNAs are defined as mitrons [35,36] first identified in Drosophila and C. elegans [36], and also found in mammals [37]. Another example of alternative miRNA pathways is that RNA polymerase III (Pol III) instead of Pol II transcribes some miRNAs [2], especially those residing upstream of Alu sequences [38]. Alu sequences or Alu elements are abundant mobile elements especially found in the primate genomes [39].
3.3 RNA interference is the central mechanism for gene regulation by miRNAs and small interfering RNA
MicroRNAs and siRNA regulate and control gene expression through RNAi [6,33]. Although siRNAs have biochemically indistinguishable mature forms of ssRNAs from those of miRNAs, the siRNA biogenesis pathway to its mature form is different from that of miRNA [2]. Dicer processes siRNA precursors, such as long dsRNAs or small hairpin RNAs (shRNAs), and cleaves them into a ~22 nt dsRNA with 2-nt 3’ overhangs [2,40,33]. This dsRNA form of siRNA is essentially equivalent with the miRNA:miRNA* duplex.
Major functionalities of siRNAs and miRNAs are that siRNAs are defenders against foreign or invasive nucleic acid molecules such as viruses, transposons, and transgenes [6], whereas miRNAs are regulators of endogenous protein-coding genes [33]. Moreover, exogenous siRNAs are widely used in gene knockdown experiments, and they are potentially useful for gene therapy. Exogenous siRNAs are directly introduced into the cytoplasm or taken up from the environment [6,33].
The central mechanism of RNAi is that si/miRNAs loaded in RISC act like guides to bind the sites of their target mRNAs. The RISCs loaded with miRNAs are called miRISCs, whereas the RISCs with siRNAs are called siRISCs [33]. RNAi has several different gene silencing modes (Fig. 3.2). The common gene silencing mode for siRNAs and plant miRNAs is cleaving mRNAs that have near-perfect complementary sites with the si/miRNAs (Fig. 3.2)[2]. Two known gene silencing modes for animal miRNAs are transcriptional repression and mRNA degradation (Fig. 3.2). In either mode, miRNAs mainly target mRNAs that have partial complementary sites on their 3’ UTRs [2]. Although translational repression was initially thought as the major regulatory model of animal miRNAs, a recent study used ribosome profiling assay and reported that most target genes of animal miRNAs were actually degraded [41]. The precise mechanism of this degradation is still unclear, but it is possibly associated with deadenylation, decapping, and exonucleolytic digestion of the mRNA [42,43,44].
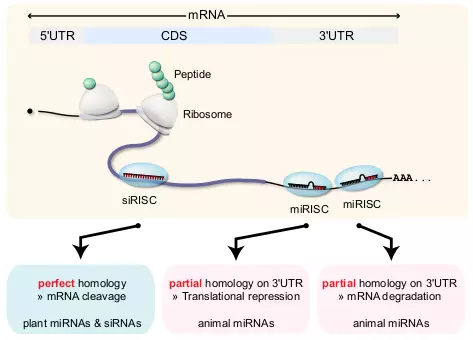
Figure 3.2. miRNA target.
The figure shows three examples of RNAi regulation by miRISC and siRISC. The long curved line represents mRNA that is separated into three regions, 5’ UTR, CDS, and 3’ UTR. Ribosomes synthesize peptides while moving through the mRNA from 5’ to 3’ direction. One siRISC binds on the CDS and two miRISCs bind on the 3’ UTR of mRNA, in this example. Plant miRNA and siRNA have nearly perfect complementary which results in mRNA cleavage. Animal miRNAs require only partial complementary, and they either repress translation or contribute to mRNA degradation.
Moreover, exogenous siRNAs are known to act like miRNAs and down-regulate numerous unintended mRNAs in the same way as miRNA gene silencing. This unintended effect is called siRNA off-targeting [45]. Considering this siRNA off-targeting is very important to design effective exogenous siRNAs.
3.4 MicroRNAs have various regulatory roles that are associated with important physiological and pathological processes
Many miRNAs play important regulatory roles by negatively controlling the expression level of mRNAs [2], and current estimates indicate that at least 60% of human protein-coding genes are under some influence of miRNAs [46]. Many miRNAs, like lin-4 and let-7, are involved in cell development processes [47]. Other experimentally validated miRNA regulatory roles can be found in many cellular processes, such as growth control, differentiation, stem cell and germline proliferation, and apoptosis [48]. However, annotations of many miRNA regulations are still poor, therefore, predicting accurate miRNA target genes is important to infer miRNA regulatory roles.
MicroRNAs are also associated with human diseases because of their wide range of gene regulatory roles [48]. Several studies revealed miRNA involved diseases, such as cancer [49,50], heart disease [51,52], DiGeorge syndrome [48], Alzheimer’s disease, and central nerve system (CNS) disorders [53]. Moreover, some viruses encode viral miRNA genes [16]. These viral miRNAs potentially regulate fundamental cellular processes both in the viruses and in their host cells [17].
3.5 Multiple properties of miRNA target recognition may enhance target efficacy in animal
Since most animal miRNAs have only partial complementary to their target mRNAs [54], miRNA targeting usually requires additional features for better target recognition. The most important feature is the seed type, which is the region for the partial complementary. The seed site contains six nucleotides from position 2 to 7 of the miRNA [55,56,54], as the position begins with 1 at the 5’ end of the miRNA. Even though the definition of the seed site is ubiquitous, the definition of the seed types is different among different studies. Figure 3.3 shows nine common seed types that are widely accepted in many studies. Seed types consist of stringent and non-stringent groups. Three seed types, 7mer-A1, 7mer-m8, and 8mer, belong to the stringent group (Fig. 3.3). These seed types have perfect Watson-Crick paring in their seed sites, and they are usually stronger than those in the non-stringent groups in terms of miRNA target recognition [54]. 7mer-A1 has an adenine (A) at position 1 of the target mRNA. An adenine at position 1 of the mRNA is known to enhance miRNA target recognition [57]. 7mer-m8 has paring at position 8. 8mer has an adenine at position 1 and paring at position 8. The non-stringent group consists of 6mer, two G:U wobble, one loop, and two bulge types (Fig. 3.3) [58]. They are less effective than the stringent group, but they are still functional since miRISC can tolerate some mismatches [59]. 6mer has perfect seed paring, whereas the other types are equivalent to 8mers except one mismatch or wobble paring. LP has a loop in the seed site. GUM has a G:U wobble site on the miRNA whereas GUT has a wobble site on the target mRNA. Similarly, BM has a bulge on the miRNA whereas BT has a bulge on the target mRNA.
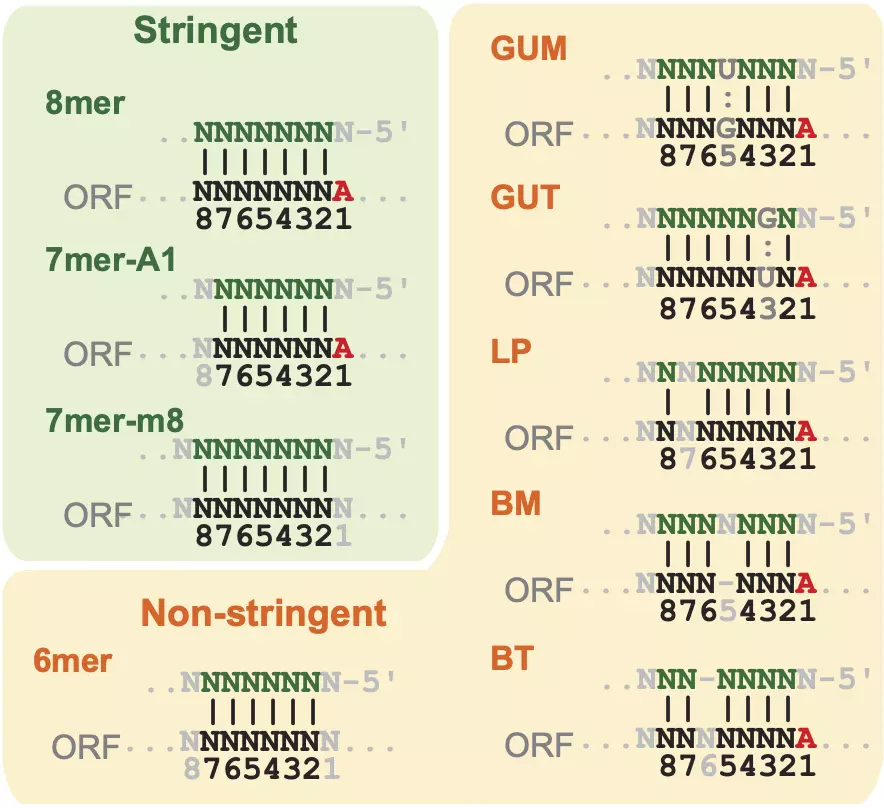
Figure 3.3. miRNA seed types.
Examples of three stringent seed types (8mer, 7mer-A1, and 7mer-m8), and six non-stringent seed types (6mer, GUM, GUT, LP, BM, and MT). The strand on the top of each seed type represents position 1-8/9 of miRNA, and the bottom strand represents target mRNAs.
Additional paring at the 3’ part of miRNAs can increase the efficacy of miRNA repression, and it can also compensate for a seed mismatch to create a functional site [54]. Three to four matches at position 13-16 for stringent seeds, and four to five matches at position 13-19 for non-stringent seeds are known as 3’ supplementary and 3’ compensatory paring, respectively [60,54].
Many target sites are well conserved among closely related species [46]. However, there are many approaches to define “well conserved” targets. For instance, some use perfect seed matches among several species [61,62,63], whereas others use pre-defined conservation scores calculated by global phylogenetic analysis [64,65]. Moreover, even though many targets are well conserved, some targets are also species-specific. For instance, one study predicted that about 30% of all experimentally validated targets are poorly conserved [66].
The site accessibility of miRISC can be measured by computing the secondary structure of target sites through minimum free energy approaches. The site accessibility is potentially a very strong feature to predict true miRNA target sites because it is directly linked to target recognition. However, the precise mechanism of miRISC access on its targets is unknown, and developing a precise prediction model with mRNA secondary structure calculation usually requires huge computational power. Some models used elaborate two step approaches with the first step as initial forming of miRNA:mRNA complex, and the second step as hybrid elongation to form the complete miRNA:mRNA complex [65,67]. An alternative feature to site accessibility is to measure the occurrence of AU rich elements both upstream and downstream of the seed site [60]. Thermodynamic stabilities of AU base pairs are much lower than that of GC base pairs, and it can be a reason that sites surrounded by AU-rich context has better site accessibility for miRISCs. Moreover, this AU rich approach is computationally inexpensive with a good prediction performance [60]. Scoring AU context is a more reliable measurement of target accessibility than any other currently available models with mRNA secondary structure calculation [54].
Multiple target sites enhance miRNA target efficacy [68]. Although the general effect of multiple sites is additive, the effect can be synergistic when two miRNA targets are within optimal distance. One reported such optimal distance is defined as two seed sites separated by between 13 and 35 nt [69]. For example, a gene with two 7mers within optimal distance is more down-regulated than a gene with a single 8mer. However, the effect is not apparent when two 7mers are not within optimal distance. In this case, a gene with 8mer is more down-regulated than a gene with two 7mers [54].
To summarize miRNA target recognition and efficacy, most animal miRNAs bind on the 3’ region of their target mRNAs by base paring. The seed site, which is located at position 2 to 7 of the miRNA, is usually used for this base paring, and the seed type tends to be one of the most important features. There are also other additional features that can contribute to target recognition and efficacy, such as 3’ additional paring, site conservation, site accessibility, and cooperability of multiple target sites.
3.6 MicroRNA binding also occurs outside of the 3’ UTR
Although the precise regulatory mechanism of miRISC’s binding on the 3’ UTR is unclear, it may cause deadenylation, decapping, and exonucleolytic digestion of the mRNA [42,43,44]. Moreover, miRISCs bind other regions that reside outside of 3’ UTRs, such as CDS [70], 5’ UTR [71,70], and promoter regions [72,73].
Many miRNAs bind CDS regions; however, most of the sites are likely non-functional because ribosomes seem to detach miRISCs from their binding site while moving along the CDS region for translation [2]. Nonetheless, there are several evidences that some miRNA target sites in CDS are functional. One example is that rare codons in CDS regions tend to make ribosomes stalled, and miRNA target sites right after these codons can be functional [74]. Moreover, some target sites experimentally validated in CDS tend to have one very strong site [75,76], or multiple sites within optimal distance [77,78].
Some miRNAs also target 5’ UTR regions, though it is much less common than 3’ UTR and CDS regions [54]. One intriguing class of miRNA targets involved in 5’ UTR is miBridge targets [71]. The miBridge target is a miRNA target site that has a normal seed site on the 3’ UTR and a 5’ portion paring on the 5’ UTR simultaneously. One possible explanation for the regulation of miBridge is that miBridge is involved in the translational initiation by preventing ribosome scanning through the 5’ UTR [71].
MicroRNA regulation can also occur inside the nucleus despite that miRNA’s major regulatory roles are in the cytoplasm [79,80]. The miRNA regulation in the nucleus is likely at the transcriptional level rather than the translational level. Several studies reported that miRNAs cause transcriptional silencing by targeting promoter regions [72,73].
3.7 Some of coding and non-coding pairs of cis-NATs potentially may have regulatory interactions with miRNAs
A complex locus is a locus that contains several genes that interact among each other [81]. Two major classes of complex loci are cis-natural antisense transcripts (cis-NATs) and bi-directionally promoters (Fig. 3.4) [81]. Many complex loci have important regulatory roles, even though the precise mechanism is unknown [82,83].
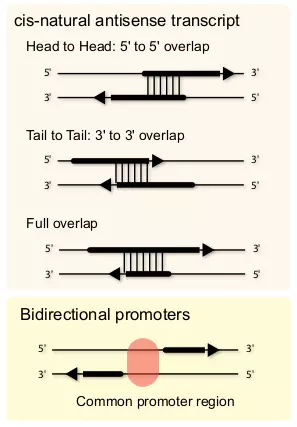
Figure 3.4. Complex loci.
Complex loci consist of multiple genes that interact among each other. Two major classes of complex loci are cis-NATs and bi-directional promoters. Cis-NATs can be divided into three categories depending on the directions of the overlaps. “Head to Head” is that sense and antisense transcripts are partially overlapped on their 5’ ends. “Tail to Tail” is that sense and antisense transcripts are partially overlapped on their 3’ ends. “Full overlap” is that one transcript is fully overlapped with the other transcript. Bi-directional promoters reside between two genes arranged head-to-head on opposite strands with less than 1000 base pairs separating their transcription start sites [84].
Cis-NATs is a sense-antisense pair of transcripts that are partially overlapping in the same locus [85]. Cis-NATs are relatively common in many species, and they are quite abundant in human [86,81,87]. Three major classes, or orientations, of cis-NATs are “head to head”, “tail to tail”, and “full overlap” (Fig. 3.4) [88]. Although the molecular mechanism of cis-NAT regulation is poorly understood, three models may explain the potential cis-NAT regulation [88]. The first model is the transcriptional collision model [88], which can be the main mechanism for “head to head” cis-NATs. The second model is the dsRNA formation model of sense and antisense transcripts [88]. One study showed that an endogenous ncRNA derived from a cis-NAT pair regulates its anti-sense transcript of a protein coding gene in plants [89]. The third model is that cis-NATs are involved in epigenetic regulation. This model is based on the evidence that some ncRNAs are involved in the modification of chromatin structure and DNA methylation in the promoter region [90,91].
Bi-directional promoters reside between two genes arranged head-to-head on opposite strands with less than 1000 base pairs separating their transcription start sites [84]. Many pairs from bi-directional promoters are co-expressed, but some are antiregulated [84]. Bi-directional promoters are abundant; for instance, they represent approximately 10% of all the genes in human [84].
The main objective of the third sub-goal: miRNA and other ncRNAs is to investigate potential miRNA interactions with other ncRNAs. Of these classes of complex loci, some miRNAs potentially interact with ncRNA:mRNA pairs of cis-NATs. In this case, miRNAs indirectly regulate the expression of the mRNAs in cis-NATs through directly regulating their paired ncRNAs (Fig. 3.5). Bi-directional promoters may also have ncRNA:mRNA pairs that potentially involve miRNAs regulation. Annotated data of bi-directional promoters are available for mRNA:mRNA pairs [81], but there are few reliable data for ncRNA:mRNA pairs of bi-directional promoters. Therefore, we excluded bi-directional promoters and focused on cis-NATs, especially on ncRNA:mRNA pairs of cis-NATs, in the third sub-goal: miRNA and other ncRNAs.
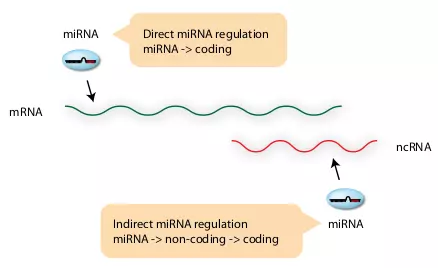
Figure 3.5. miRNA regulation on cis-NAT.
The figure illustrates two different potential modes of miRNA regulation on cis-NATs. Direct miRNA regulation is a normal regulatory mode of miRNAs, in which miRNA binds on the 3’ UTR of mRNA. Indirect mode is that miRNA regulates the protein coding mRNA in cis-NAT indirectly through binding the non-coding transcript.
3.8 Chromatin associated RNAs are potentially associated with the modification of chromatin structure
Chromatin associated RNAs (CARs) are experimentally validated non-coding RNAs that can bind a part of the chromatin directly [92]. Since CARs affect their host and neighboring genes [92], CARs can be seen as a class of complex loci. CARs are likely involved in the regulation of chromatin structure by recruiting chromatin-modifying complexes (Fig. 3.6). This scenario is supported by the evidence that long ncRNAs regulate chromatin modification by guiding chromatin remodeling complexes to specific genome loci [93,94]. In addition to long ncRNAs, RNAi is also known to have roles in the regulation of chromatin structure. For instance, in fission yeast, CARs serve as assembly platform to the RNA-induced initiation of transcriptional gene-silencing (RITS) complex. In this case of fission yeast, siRNAs associate with AGO1 and guide the RITS complex to CARs [91].
Similar to ncRNA:mRNA pairs of cis-NATs, CARs potentially have interactions with miRNAs, though there is currently no strong evidence to support this. Therefore, we chose cis-NATs and CARs to investigate their potential interactions with miRNAs in the third sub-goal: miRNA and other ncRNAs.
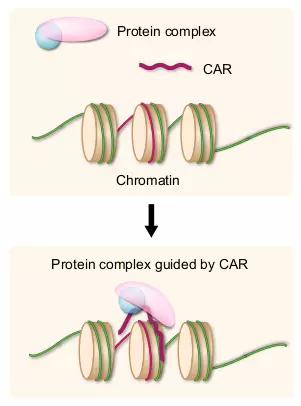
Figure 3.6. CARs.
Upper panel shows a protein complex, a CAR, and nucleosomes. The complementary DNA region to the CAR is indicated in red. Lower panel shows one example of the CAR regulation to the chromatin. The CAR acts as a guide for the protein complex that can modify the chromatin structure.
References
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993;75:843–54. https://doi.org/10.1016/0092-8674(93)90529-y.
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–97. https://doi.org/10.1016/s0092-8674(04)00045-5.
- Fire A, Xu SQ, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998;391:806–11. https://doi.org/10.1038/35888.
- Hamilton AJ, Baulcombe DC. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 1999;286:950–2. https://doi.org/10.1126/science.286.5441.950.
- Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001;411:494–8. https://doi.org/10.1038/35078107.
- Mello CC, Conte D. Revealing the world of RNA interference. Nature 2004;431:338–42. https://doi.org/10.1038/nature02872.
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, et al. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 2000;403:901–6. https://doi.org/10.1038/35002607.
- Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kuroda MI, Maller B, et al. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 2000;408:86–9. https://doi.org/10.1038/35040556.
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science 2001;294:853–8. https://doi.org/10.1126/science.1064921.
- Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in caenorhabditis elegans. Science 2001;294:858–62. https://doi.org/10.1126/science.1065062.
- Lee RC, Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science 2001;294:862–4. https://doi.org/10.1126/science.1065329.
- Griffiths-Jones S. The microRNA registry. Nucleic Acids Research 2004;32:109D–111. https://doi.org/10.1093/nar/gkh023.
- Griffiths-Jones S. miRBase: the MicroRNA sequence database. MicroRNA Protocols, vol. 342, Humana Press; 2006, p. 129–38. https://doi.org/10.1385/1-59745-123-1:129.
- Griffiths-Jones S, Saini HK, Dongen S van, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Research 2007;36:D154–D158. https://doi.org/10.1093/nar/gkm952.
- Kozomara A, Griffiths-Jones S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Research 2010;39:D152–D157. https://doi.org/10.1093/nar/gkq1027.
- Nair V, Zavolan M. Virus-encoded microRNAs: novel regulators of gene expression. Trends in Microbiology 2006;14:169–75. https://doi.org/10.1016/j.tim.2006.02.007.
- Boss IW, Plaisance KB, Renne R. Role of virus-encoded microRNAs in herpesvirus biology. Trends in Microbiology 2009;17:544–53. https://doi.org/10.1016/j.tim.2009.09.002.
- Lee Y, Kim M, Han J, Yeom K-H, Lee S, Baek SH, et al. MicroRNA genes are transcribed by RNA polymerase II. The EMBO Journal 2004;23:4051–60. https://doi.org/10.1038/sj.emboj.7600385.
- Lee Y, Jeon K, Lee J-T, Kim S, Kim VN. MicroRNA maturation: stepwise processing and subcellular localization. The EMBO Journal 2002;21:4663–70. https://doi.org/10.1093/emboj/cdf476.
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003;425:415–9. https://doi.org/10.1038/nature01957.
- Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature 2004;432:231–5. https://doi.org/10.1038/nature03049.
- Gregory RI, Yan K-ping, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, et al. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004;432:235–40. https://doi.org/10.1038/nature03120.
- Han J, Lee Y, Yeom K-H, Kim Y-K, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes & Development 2004;18:3016–27. https://doi.org/10.1101/gad.1262504.
- Landthaler M, Yalcin A, Tuschl T. The human DiGeorge syndrome critical region gene 8 and its d. Melanogaster homolog are required for miRNA biogenesis. Current Biology 2004;14:2162–7. https://doi.org/10.1016/j.cub.2004.11.001.
- Sætrom P, Snøve O, Nedland M, Grünfeld TB, Lin Y, Bass MB, et al. Conserved MicroRNA characteristics in mammals. Oligonucleotides 2006;16:115–44. https://doi.org/10.1089/oli.2006.16.115.
- Zeng Y, Yi R, Cullen BR. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proceedings of the National Academy of Sciences 2003;100:9779–84. https://doi.org/10.1073/pnas.1630797100.
- Batuwita R, Palade V. microPred: effective classification of pre-miRNAs for human miRNA gene prediction. Bioinformatics 2009;25:989–95. https://doi.org/10.1093/bioinformatics/btp107.
- Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of MicroRNA precursors. Science 2004;303:95–8. https://doi.org/10.1126/science.1090599.
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes & Development 2003;17:3011–6. https://doi.org/10.1101/gad.1158803.
- Tomari Y, Zamore PD. Perspective: machines for RNAi. Genes & Development 2005;19:517–29. https://doi.org/10.1101/gad.1284105.
- Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nature Reviews Molecular Cell Biology 2005;6:376–85. https://doi.org/10.1038/nrm1644.
- Fagard M, Boutet S, Morel J-B, Bellini C, Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proceedings of the National Academy of Sciences 2000;97:11650–4. https://doi.org/10.1073/pnas.200217597.
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009;136:642–55. https://doi.org/10.1016/j.cell.2009.01.035.
- Hammond SM, Bernstein E, Beach D, Hannon GJ. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 2000;404:293–6. https://doi.org/10.1038/35005107.
- Okamura K, Hagen JW, Duan H, Tyler DM, Lai EC. The mirtron pathway generates microRNA-class regulatory RNAs in Drosophila. Cell 2007;130:89–100. https://doi.org/10.1016/j.cell.2007.06.028.
- Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature 2007;448:83–6. https://doi.org/10.1038/nature05983.
- Berezikov E, Chung W-J, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Molecular Cell 2007;28:328–36. https://doi.org/10.1016/j.molcel.2007.09.028.
- Borchert GM, Lanier W, Davidson BL. RNA polymerase III transcribes human microRNAs. Nature Structural & Molecular Biology 2006;13:1097–101. https://doi.org/10.1038/nsmb1167.
- Batzer MA, Deininger PL. Alu repeats and human genomic diversity. Nature Reviews Genetics 2002;3:370–9. https://doi.org/10.1038/nrg798.
- Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes & Development 2002;16:948–58. https://doi.org/10.1101/gad.981002.
- Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 2010;466:835–40. https://doi.org/10.1038/nature09267.
- Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes & Development 2006;20:1885–98. https://doi.org/10.1101/gad.1424106.
- Giraldez AJ, Mishima Y, Rihel J, Grocock RJ, Dongen SV, Inoue K, et al. Zebrafish MiR-430 promotes deadenylation and clearance of maternal mRNAs. Science 2006;312:75–9. https://doi.org/10.1126/science.1122689.
- Wu L, Fan J, Belasco JG. MicroRNAs direct rapid deadenylation of mRNA. Proceedings of the National Academy of Sciences 2006;103:4034–9. https://doi.org/10.1073/pnas.0510928103.
- Jackson AL, Bartz SR, Schelter J, Kobayashi SV, Burchard J, Mao M, et al. Expression profiling reveals off-target gene regulation by RNAi. Nature Biotechnology 2003;21:635–7. https://doi.org/10.1038/nbt831.
- Friedman RC, Farh KK-H, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research 2008;19:92–105. https://doi.org/10.1101/gr.082701.108.
- Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nature Reviews Molecular Cell Biology 2008;9:219–30. https://doi.org/10.1038/nrm2347.
- Alvarez-Garcia I, Miska EA. MicroRNA functions in animal development and human disease. Development 2005;132:4653–62. https://doi.org/10.1242/dev.02073.
- He L, Thomson JM, Hemann MT, Hernando-Monge E, Mu D, Goodson S, et al. A microRNA polycistron as a potential human oncogene. Nature 2005;435:828–33. https://doi.org/10.1038/nature03552.
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature 2005;435:834–8. https://doi.org/10.1038/nature03702.
- Chen J-F, Murchison EP, Tang R, Callis TE, Tatsuguchi M, Deng Z, et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proceedings of the National Academy of Sciences 2008;105:2111–6. https://doi.org/10.1073/pnas.0710228105.
- Zhao Y, Ransom JF, Li A, Vedantham V, Drehle M von, Muth AN, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell 2007;129:303–17. https://doi.org/10.1016/j.cell.2007.03.030.
- Maes O, Chertkow H, Wang E, Schipper H. MicroRNA: implications for alzheimer disease and other human CNS disorders. Current Genomics 2009;10:154–68. https://doi.org/10.2174/138920209788185252.
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell 2009;136:215–33. https://doi.org/10.1016/j.cell.2009.01.002.
- Lewis BP, Shih I-hung, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian MicroRNA targets. Cell 2003;115:787–98. https://doi.org/10.1016/s0092-8674(03)01018-3.
- Brennecke J, Stark A, Russell RB, Cohen SM. Principles of MicroRNA-target recognition. PLoS Biology 2005;3:e85. https://doi.org/10.1371/journal.pbio.0030085.
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are MicroRNA targets. Cell 2005;120:15–20. https://doi.org/10.1016/j.cell.2004.12.035.
- Gaidatzis D, Nimwegen E van, Hausser J, Zavolan M. Inference of miRNA targets using evolutionary conservation and pathway analysis. BMC Bioinformatics 2007;8:69. https://doi.org/10.1186/1471-2105-8-69.
- Ellwanger DC, Büttner FA, Mewes H-W, Stümpflen V. The sufficient minimal set of miRNA seed types. Bioinformatics 2011;27:1346–50. https://doi.org/10.1093/bioinformatics/btr149.
- Grimson A, Farh KK-H, Johnston WK, Garrett-Engele P, Lim LP, Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Molecular Cell 2007;27:91–105. https://doi.org/10.1016/j.molcel.2007.06.017.
- Krek A, Grün D, Poy MN, Wolf R, Rosenberg L, Epstein EJ, et al. Combinatorial microRNA target predictions. Nature Genetics 2005;37:495–500. https://doi.org/10.1038/ng1536.
- Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in Drosophila. Genome Biology 2003;5:R1. https://doi.org/10.1186/gb-2003-5-1-r1.
- Lall S, Grün D, Krek A, Chen K, Wang Y-L, Dewey CN, et al. A genome-wide map of conserved MicroRNA targets in C. Elegans. Current Biology 2006;16:460–71. https://doi.org/10.1016/j.cub.2006.01.050.
- Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biology 2010;11:R90. https://doi.org/10.1186/gb-2010-11-8-r90.
- Kertesz M, Iovino N, Unnerstall U, Gaul U, Segal E. The role of site accessibility in microRNA target recognition. Nature Genetics 2007;39:1278–84. https://doi.org/10.1038/ng2135.
- Sethupathy P, Megraw M, Hatzigeorgiou AG. A guide through present computational approaches for the identification of mammalian microRNA targets. Nature Methods 2006;3:881–6. https://doi.org/10.1038/nmeth954.
- Long D, Lee R, Williams P, Chan CY, Ambros V, Ding Y. Potent effect of target structure on microRNA function. Nature Structural & Molecular Biology 2007;14:287–94. https://doi.org/10.1038/nsmb1226.
- John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biology 2004;2:e363. https://doi.org/10.1371/journal.pbio.0020363.
- Saetrom P, Heale BSE, Snøve O, Aagaard L, Alluin J, Rossi JJ. Distance constraints between microRNA target sites dictate efficacy and cooperativity. Nucleic Acids Res 2007;35:2333–42. https://doi.org/10.1093/nar/gkm133.
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, et al. Transcriptome-wide identification of RNA-binding protein and MicroRNA target sites by PAR-CLIP. Cell 2010;141:129–41. https://doi.org/10.1016/j.cell.2010.03.009.
- Lee I, Ajay SS, Yook JI, Kim HS, Hong SH, Kim NH, et al. New class of microRNA targets containing simultaneous 5’-UTR and 3’-UTR interaction sites. Genome Research 2009;19:1175–83. https://doi.org/10.1101/gr.089367.108.
- Kim DH, Saetrom P, Snove O, Rossi JJ. MicroRNA-directed transcriptional gene silencing in mammalian cells. Proceedings of the National Academy of Sciences 2008;105:16230–5. https://doi.org/10.1073/pnas.0808830105.
- Place RF, Li L-C, Pookot D, Noonan EJ, Dahiya R. MicroRNA-373 induces expression of genes with complementary promoter sequences. Proceedings of the National Academy of Sciences 2008;105:1608–13. https://doi.org/10.1073/pnas.0707594105.
- Gu S, Jin L, Zhang F, Sarnow P, Kay MA. Biological basis for restriction of microRNA targets to the 3′ untranslated region in mammalian mRNAs. Nature Structural & Molecular Biology 2009;16:144–50. https://doi.org/10.1038/nsmb.1552.
- Duursma AM, Kedde M, Schrier M, Sage C le, Agami R. miR-148 targets human DNMT3b protein coding region. RNA 2008;14:872–7. https://doi.org/10.1261/rna.972008.
- Elcheva I, Goswami S, Noubissi FK, Spiegelman VS. CRD-BP protects the coding region of βTrCP1 mRNA from miR-183-mediated degradation. Molecular Cell 2009;35:240–6. https://doi.org/10.1016/j.molcel.2009.06.007.
- Forman JJ, Legesse-Miller A, Coller HA. A search for conserved sequences in coding regions reveals that the let-7 microRNA targets Dicer within its coding sequence. Proceedings of the National Academy of Sciences 2008;105:14879–84. https://doi.org/10.1073/pnas.0803230105.
- Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008;455:1124–8. https://doi.org/10.1038/nature07299.
- Hwang H-W, Wentzel EA, Mendell JT. A hexanucleotide element directs MicroRNA nuclear import. Science 2007;315:97–100. https://doi.org/10.1126/science.1136235.
- Politz JCR, Zhang F, Pederson T. MicroRNA-206 colocalizes with ribosome-rich regions in both the nucleolus and cytoplasm of rat myogenic cells. Proceedings of the National Academy of Sciences 2006;103:18957–62. https://doi.org/10.1073/pnas.0609466103.
- Engström PG, Suzuki H, Ninomiya N, Akalin A, Sessa L, Lavorgna G, et al. Complex loci in human and mouse genomes. PLoS Genetics 2006;2:e47. https://doi.org/10.1371/journal.pgen.0020047.
- Duhig T, Ruhrberg C, Mor O, Fried M. The human surfeit locus. Genomics 1998;52:72–8. https://doi.org/10.1006/geno.1998.5372.
- Holmes R, Williamson C, Peters J, Denny P, Group RIKENGER, Members GSL, et al. A comprehensive transcript map of the mouse Gnas imprinted complex. Genome Research 2003;13:1410–5. https://doi.org/10.1101/gr.955503.
- Trinklein ND, Aldred SF, Hartman SJ, Schroeder DI, Otillar RP, Myers RM. An abundance of bidirectional promoters in the human genome. Genome Research 2003;14:62–6. https://doi.org/10.1101/gr.1982804.
- Wang X-J, Gaasterland T, Chua N-H. Genome-wide prediction and identification of cis-natural antisense transcripts in Arabidopsis thaliana. Genome Biology 2005;6:R30. https://doi.org/10.1186/gb-2005-6-4-r30.
- Chen J, Sun M, Kent WJ, Huang X, Xie H, Wang W, et al. Over 20% of human transcripts might form sense-antisense pairs. Nucleic Acids Research 2004;32:4812–20. https://doi.org/10.1093/nar/gkh818.
- Zhang Y, Liu XS, Liu Q-R, Wei L. Genome-wide in silico identification and analysis of cis natural antisense transcripts (cis-NATs) in ten species. Nucleic Acids Research 2006;34:3465–75. https://doi.org/10.1093/nar/gkl473.
- Osato N, Suzuki Y, Ikeo K, Gojobori T. Transcriptional interferences in cis natural antisense transcripts of humans and mice. Genetics 2007;176:1299–306. https://doi.org/10.1534/genetics.106.069484.
- Borsani O, Zhu J, Verslues PE, Sunkar R, Zhu J-K. Endogenous siRNAs derived from a pair of natural cis-antisense transcripts regulate salt tolerance in arabidopsis. Cell 2005;123:1279–91. https://doi.org/10.1016/j.cell.2005.11.035.
- Tufarelli C, Stanley JAS, Garrick D, Sharpe JA, Ayyub H, Wood WG, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nature Genetics 2003;34:157–65. https://doi.org/10.1038/ng1157.
- Beisel C, Paro R. Silencing chromatin: comparing modes and mechanisms. Nature Reviews Genetics 2011;12:123–35. https://doi.org/10.1038/nrg2932.
- Mondal T, Rasmussen M, Pandey GK, Isaksson A, Kanduri C. Characterization of the RNA content of chromatin. Genome Research 2010;20:899–907. https://doi.org/10.1101/gr.103473.109.
- Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nature Reviews Genetics 2009;10:155–9. https://doi.org/10.1038/nrg2521.
- Whitehead J, Pandey GK, Kanduri C. Regulation of the mammalian epigenome by long noncoding RNAs. Biochimica Et Biophysica Acta (BBA) - General Subjects 2009;1790:936–47. https://doi.org/10.1016/j.bbagen.2008.10.007.